- Home page
- MDR 2017/745
- Link Normativi / Regulatory Link
- Esclusione di responsabilità / Disclaimer Clause
- TEACHING AIDS - ENGLISH
- РУССКИЙ ЯЗЫК
- CREDITI / CREDITS
- Assistenza Legale / Legal Assistance
- Alimentazione
- CONVIVIUM: RECIPES & COOKING CONFIDENCE FROM ITALY
- Contatti / Contacts
- DIZIONARI/DICTIONARY
- Pubblicazioni Scientifiche / Scientific Pubblications
- DAD/DDI 🖳🖰 MATERIALI PER LA DIDATTICA
mercoledì 20 agosto 2014
martedì 19 agosto 2014
lunedì 18 agosto 2014
lunedì 11 agosto 2014
50 - RECUPERO FISICO NELLE PRESTAZIONI SPORTIVE CON LO STRETCHING MANDIBOLARE (informazioni di sintesi)
Daniele Tonlorenzi – Luca Martinelli
RECUPERO
FISICO NELLE PRESTAZIONI SPORTIVE
CON LO
STRETCHING MANDIBOLARE
(informazioni
di sintesi)
Pubblicazione n. 50 – 07 Agosto 2014
1.PREMESSA
Nel soggetto sportivo, durante la gara o
l’allenamento si manifesta uno stress psico-fisico che aumenta
-
Frequenza del battito cardiaco;
-
Pressione sanguigna;
-
Tono muscolare;
-
Impegno mentale.
Solo
alla fine della prestazione sportiva (gara o allenamento) i parametri cardiaci
e i parametri pressori tornano nella norma pre-stress, questo è possibile
grazie all’attività di recupero.
Se
il recupero non è ottimale permane la stanchezza, si ha una riduzione
dell’agilità e dei riflessi, si possono manifestare contratture muscolari con
possibili complicazioni muscolo-tendinei (Es. pubalgia) e/o ossei (Es.
microfratture), riduzione delle capacità di performance.
In
queste condizioni il soggetto si espone anche a un elevato rischio di incidente
sportivo.
2 AUMENTARE
LE PERFORMANCE
Ogni sport, per potenziare le
prestazioni, ha il suo metodo di
preparazione atletica, i suoi tempi, i suoi luoghi, di certo tutti hanno in
comune una cosa: la necessità di rilassamento per ottenere il massimo ristoro.
Per
aumentare le performance quindi, una strada da percorrere con fiducia di avere
dei buoni risultati è indubbiamente quella di ottenere il più possibile un
recupero dei parametri di frequenza cardiaca, pressione sanguigna, tono
muscolare e impegno mentale.
Studi e test hanno dimostrato che
l’attività di stretching mandibolare, mai utilizzata fino ad oggi nello sport,
porta dei benefici tanto oggettivi quanto inaspettati.
Se con la mente torniamo a tante immagini dello sport ci ricorderemo
immediatamente come gli sportivi nel massimo della performance stringono i
denti, li serrano quasi in maniera spasmodica.
Non è semplicemente un atteggiamento
personale, il serrare i denti per 1 minuto permette all’organismo di potenziare
i meccanismi dello stress e di far aumentare la pressione arteriosa media di
5,5 mm hg. (http://www.ncbi.nlm.nih.gov/pubmed/277577).
Questo fatto concede al soggetto di
esprimere al suo massimo la performance sportiva.
Altrettanto chiara è l’immagine della bocca aperta del
piacevole sbadiglio e così come la bocca serrata si associa al concetto di
stress, la bocca aperta, la bocca che sbadiglia è associata al concetto di
relax.
Questo studio è stato presentato nella
tesi sperimentale di COPPI, ERIKA Studio del riflesso trigemino-cardiaco nel
controllo della pressione arteriosa e della frequenza cardiaca (http://etd.adm.unipi.it/t/etd-04072010-114646/)
è stato fatto con il prototipo
sperimentale del dispositivo medico Spring
device ed è stato pubblicato (http://www.ncbi.nlm.nih.gov/pubmed/23479456)
Da questa immagine si percepisce che
lo stretching mandibolare è un meccanismo d’azione che già trova impiego in
natura senza che nemmeno che ne accorgiamo.
3 GLI
STRUMENTI PER AUMENTARE LE PERFORMANCE
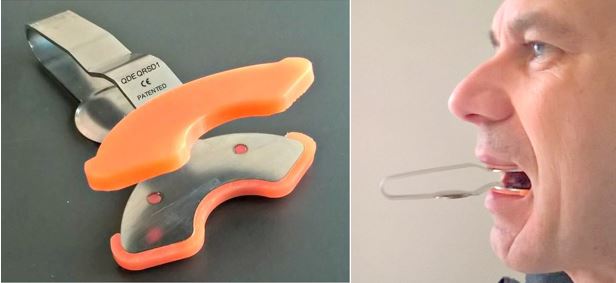
Per gli studi e test
sullo stretching mandibolare è stato impiegato lo Spring Device (Foto 1 e 2), uno strumento che permette di eseguire
un estensione mandibolare in associazione a movimenti masticatori, in pratica
un attività di stretching mandibolare. Test hanno dimostrato che l’impiego di
questo strumento, ovvero l’attività di stretching mandibolare, permette un
abbassamento della frequenza e pressione cardiaca (nell'uomo) e che fa
aumentare la quantità di sangue che arriva al cervello (nel vertebrato), è
quindi ipotizzabile che questa attività favorisca anche il recupero mentale,
spesso di fondamentale importanza nella prestazione sportiva.
Foto 1 Foto 2
Dispositivo medico Spring Device Spring device nella bocca di un tester
Dispositivo medico Spring Device Spring device nella bocca di un tester
4
CONCLUSIONI
Recenti studi hanno
dimostrato che l’impiego dello stretching mandibolare dinamico permette
all’organismo di recuperare velocemente i parametri fisiologici che l’atleta ha
prima della prestazione.
L’impiego del
dispositivo medico Spring Device permette di eseguire lo stretching mandibolare
e quindi permette all’atleta di recuperare in fretta.
Lo Spring Device
agisce attraverso meccanismi totalmente naturali per cui non è considerabile in
nessuna maniere doping sportivo.
5 BIBLIOGRAFIA
1
2
3
COPPI,
ERIKA Studio del riflesso
trigemino-cardiaco nel controllo della pressione arteriosa e della frequenza
cardiaca Tesi di Laurea Specialistica - Universita’ di Pisa – URN etd-04072010-114646 – 2010
4
Se l’atleta è più rilassato migliorano le performance? Si
può rispondere misurando? – Tonlorenzi
D, D’angelo M, Ravera F, Traina G, Brunelli M - http://www.danieletonlorenzi.it/category/sport/ oppure http://www.slideshare.net/DanieleTonlorenzi/sport-35154369?ref=http://www.danieletonlorenzi.it/category/sport/
5
Analisi delle modificazioni
prestazionali indotte dall’uso dello Spring Device su ciclisti di alto livello.
Un esperimento – Ravera F
http://www.danieletonlorenzi.it/category/sport/ oppure http://www.slideshare.net/DanieleTonlorenzi/sport-35154369?ref=http://www.danieletonlorenzi.it/category/sport/
6
12 Lo stretching Mandibolare – Tonlorenzi D, Martinelli L
- http://martinelliluca.blogspot.it/2012/11/12-lo-stretching-mandibolare.html
7
venerdì 8 agosto 2014
SUPERATE 360.000 VISITE
Cari lettori: colleghi, studenti, docenti, professionisti,
aziende ed enti pubblici che seguite questo blog anche attraverso google plus.
Il numero di visite ricevute rappresenta il successo di
tutti noi che insieme leggendo, discutendo, domandando e anche criticando,
abbiamo prodotto documenti tecnici, schede, statistiche, notizie sulla
sicurezza dei prodotti, sulle norme e leggi utili a tutti noi sia per lo studio
che nella professione.
Questo è uno spazio nato spontaneamente, non soltanto mio ma
anche vostro. Insieme lo miglioreremo per trarne il maggior vantaggio possibile
per ognuno di noi ma anche per il piacere di condividere le nostre diverse
conoscenze.
Grazie infinite alle persone che fanno parte di questo
progetto e lo seguono con passione.
Rimango a disposizione di tutti coloro i
quali vorranno unirsi a noi, invitandovi a contattarmi al mio indirizzo di
posta elettronica martinellimartinelli@live.com
Luca Martinelli

giovedì 7 agosto 2014
THE GLOBAL MEDICAL DEVICE NOMENCLATURE (GMDN)
The Global Medical
Device Nomenclature (GMDN) is a system of internationally agreed terms used to
identify medical devices.
It is used by regulators, hospitals and manufacturers to identify medical devices that are of the same generic type.
This supports market
surveillance, adverse event reporting, product recall and other healthcare
management activities.
The GMDN is a
system of internationally agreed generic descriptors used to identify all
medical device products.
Such products include those used in the diagnosis, prevention, monitoring,
treatment or alleviation of disease or injury in humans.
The main purpose of the GMDN is to provide health authorities and
regulators, health care providers, medical device manufacturers and suppliers,
conformity assessment bodies and others with a single generic naming system
that will support patient safety.
The GMDN is used for:
- Data
exchange between manufacturers, regulators and healthcare authorities;
- Exchange
of post-market vigilance information;
- Supporting inventory control in hospitals;
- Purchasing and supply chain management.
Medical device experts from around the world (manufacturers, healthcare
authorities and regulators) compiled the GMDN, based on the international
standard ISO 15225.
The work was mandated by the European Commission in order to provide the
necessary tool to carry out the implementation of the Medical Devices
Directive, including the European databank for medical devices, Eudamed.
The GMDN meets the need to identify medical devices at the global level, as
identified by the Global Harmonization Task Force (GHTF) and is now used by
over 50 national medical device regulators.
The GMDN is managed by the GMDN Agency, a non-profit organization, which
reports to its Board of Trustees, that represents medical device regulators and
industry.
The GMDN Agency updates the GMDN utilizing member change requests, to add a
new device terms to the GMDN.
The decisions are made by an international expert team on how the new term
is to be described, according to ISO 15225.
The GMDN is available in English and
24 other languages.
The GMDN Agency releases new and updated GMDN terms on a daily basis, on
their interactive website, the GMDN Database.
Information in the form of a 5
digit numeric GMDN Code is cross-referenced to a precisely defined descriptive
Preferred Term, with which all specific devices have substantially similar
generic features.
All GMDN terms have the following data elements, as seen in this example:
Example of GMDN Code and terms:
GMDN® Term Name
Scalpel, single-use
GMDN® Code
47569
GMDN® Definition
A sterile, hand-held, manual surgical instrument constructed as a one-piece
handle and scalpel blade (not an exchangeable component) used by the operator
to manually cut or dissect tissue.
The blade is typically made of high-grade stainless steel alloy or carbon
steel and the handle is often made of plastic.
This is a single-use device.
The GMDN Database is priced according to organization size.
Users can register for access, apply for new terms and pay on-line.
About GMDN Membership
The GMDN Agency is responsible for maintaining the
GMDN. Access is only available to Members and membership is priced according to
organisation type and size. The membership process is completed online and
access to the database is immediate upon completion of payment which can be
done on-line or by invoice.
Members search the online database for the GMDN Term that describes their product. Each GMDN Term has a 5-digit Code that is revealed using a code credit. The GMDN Code can then be given to a regulator or customer for their intended use.
To ensure there is a GMDN Term for all medical devices, for example to meet the need for product innovation, the database is updated using change requests from Members.
Members search the online database for the GMDN Term that describes their product. Each GMDN Term has a 5-digit Code that is revealed using a code credit. The GMDN Code can then be given to a regulator or customer for their intended use.
To ensure there is a GMDN Term for all medical devices, for example to meet the need for product innovation, the database is updated using change requests from Members.
For more
information visit:
Welcome to the GMDN
The Global Medical Device Nomenclature (GMDN) is a system of internationally agreed terms used to identify medical devices.
It is used by regulators, hospitals and manufacturers to identify medical devices that are of the same generic type. This supports market surveillance, adverse event reporting, product recall and other healthcare management activities.
martedì 5 agosto 2014
AUDITS AND ASSESMENTS PERFORMED BY NOTIFIED BODIES IN THE FIELD OF MEDICAL DEVICES - UNANNOUNCED AUDITS
(For unannounced aduits see annex III)
COMMISSION RECOMMENDATION
of 24 September 2013
on the audits and assessments performed by notified bodies in the field of medical devices
(Text with EEA relevance)
(2013/473/EU)
...omississ...
HAS ADOPTED THIS RECOMMENDATION:
1. PURPOSE
To facilitate the consistent application of the conformity assessment provisions contained in Directive 90/385/EEC, Directive 93/42/EEC and Directive 98/79/EC, the notified bodies should apply the provisions of this Recommendation when they perform product assessments, quality system assessments and unannounced audits.
By providing general guidelines for such assessments and unannounced audits, this Recommendation should facilitate the work of the notified bodies as well as the Member States’ evaluation thereof. This Recommendation does not create any new rights and obligations. The legal requirements applicable to all types of devices and conformity assessments are set out in the Union legislation on medical devices.
2. GENERAL GUIDELINES FOR AUDITS AND ASSESSMENTS
The notified bodies should apply the following:
(a)
|
Where the manufacturer has applied for a design dossier examination or for a type examination (hereinafter jointly referred to as ‘product assessment’), notified bodies should verify the conformity of the device under all product related aspects referred to in Directive 90/385/EEC, Directive 93/42/EEC and Directive 98/79/EC for detecting any non-compliance of the device and should apply Annex I.
|
(b)
|
Where the manufacturer has applied for an assessment of its quality system, notified bodies should verify the conformity of the quality system with the quality-system related requirements contained in Directive 90/385/EEC, Directive 93/42/EEC and Directive 98/79/EC for detecting non-compliances of the quality system and should apply Annex II.
|
(c)
|
To verify the day-to-day compliance with legal obligations, notified bodies should, in addition to the initial, surveillance or renewal audits, visit the manufacturer or, if this is likely to ensure more efficient control, one of its subcontractors in charge of processes which are essential for ensuring compliance with legal requirements (‘critical subcontractor’) or a supplier of crucial components or of the entire devices (both: ‘crucial supplier’) without prior notice (‘unannounced audits’) in accordance with Annex III.
|
3. FOLLOW-UP
Member States should draw this Recommendation to the attention of the notified bodies in the field of medical devices and should supervise the practice of notified bodies with respect to this Recommendation. They should evaluate the notified bodies’ readiness to apply this Recommendation and in particular to perform unannounced audits when deciding on designations of bodies and on renewal or withdrawal of designations.
4. ADDRESSEES
This Recommendation is addressed to the Member States.
Done at Brussels, 24 September 2013.
For the Commission
Neven Mimica
Member of the commission
Link
ANNEX I
Product assessment
1.
|
Notified bodies should verify if the device is correctly qualified as a medical device and, in particular, whether the manufacturer has assigned a medical purpose to the device. They should furthermore verify the classification of the device and whether the manufacturer has fulfilled the applicable conformity assessment obligations. They should satisfy the obligations of consultation for certain devices that incorporate a substance which, in case used separately, may be considered to be a medicinal product, a human blood derivative or an animal tissue (1).
|
2.
|
Notified bodies should verify the compliance of the device with the relevant Essential Requirements set out in Annex 1 to Directive 90/385/EEC, Annex I to Directive 93/42/EEC and Annex I to Directive 98/79/EC and, if applicable, with the essential safety and health requirements (ESHR) set out in Directive 2006/42/EC. In the case of in vitro diagnostic medical devices, where applicable, they should also verify the compliance of the device with the common technical specifications laid down in Decision 2002/364/EC or, when duly justified, with other technical solutions of a level at least equivalent. Where doubts arise, in the framework of a design dossier examination, as to the conformity of a device, notified bodies should carry out or ask for relevant tests of the device.
|
3.
|
Notified bodies should examine the requirements regarding design and construction and the ESHR prior to examining the general requirements set out in Part I of Annex 1 to Directive 90/385/EEC, in Part A of Annex I to Directive 93/42/EEC and in Part A of Annex I to Directive 98/79/EC. They should apply special care to examine all the following aspects of the essential requirements:
|
4.
|
The examination of the general requirements should ascertain that among others the following requirements have been met:
|
5.
|
For medical devices other than in vitro diagnostic devices, notified bodies should review all relevant preclinical data, the clinical evaluation and the post-market clinical follow-up undertaken or planned by the manufacturer. They should verify that the clinical evaluation is up-to-date. They should assess the need for and the appropriateness of a post-market clinical follow-up plan (2). If no clinical investigation has been undertaken, they should verify that the device type in question and all the different types of risks linked to the device design, its materials, and its use are appropriately assessed by means of scientific literature or other existing clinical data so that no clinical investigation is needed; they should furthermore examine the special justification (3) needed for implantable devices and devices classified within class III according to Annex IX to Directive 93/42/EEC.
|
6.
|
In the case of in vitro diagnostic medical devices, notified bodies should review the performance evaluation undertaken by the manufacturer and post-market follow up undertaken or planned by the manufacturer.
|
7.
|
Notified bodies should verify all documentation related to the device’s conformity assessment. To that end, they should verify that the technical documentation is correct, consistent, relevant, up-to-date and complete (4) and that it covers all variants and trade names of the device. They should furthermore verify that the manufacturer’s device identification system and its practice of defining which devices belong to the same type ensure that the notified body’s certificates, the manufacturer’s declarations of conformity and the manufacturer’s technical documentations can unequivocally be attributed to the device examined. They should finally verify that the draft declaration of conformity contains all the necessary items.
|
8.
|
The notified body should clearly document the conclusions of its assessment and it should be clearly evidenced how the conclusions are taken into account as part of the notified body’s decision making process.
|
(1) See Section 10 of Annex 1, Section 4.3 of Annex 2 and Section 5 of Annex 3 to Directive 90/385/EEC, Section 7.4 of Annex I, Section 4.3 of Annex II and Section 5 of Annex III to Directive 93/42/EEC and Regulation (EU) No 722/2012.
(2) See Section 1.4 of Annex 7 to Directive 90/385/EEC and Section 1.1c of Annex X to Directive 93/42/EEC.
(3) See Annex 7 to Directive 90/385/EEC and Annex X to Directive 93/42/EEC.
(4) To be regarded as complete, a technical documentation should cover with appropriate depth the items listed in the document of the Global Harmonization Task Force ‘Summary Technical Documentation for Demonstrating Conformity to the Essential Principles of Safety and Performance of Medical Devices (STED)’ as well as additional items required by the European legislation or, for in vitro diagnostic medical devices, ‘Summary Technical Documentation (STED) for Demonstrating Conformity to the Essential Principles of Safety and Performance of In Vitro Diagnostic Medical Devices’ as well as additional items required by the European legislation, see for these documents http://www.imdrf.org/ghtf/ghtf-archives-sg1.asp
ANNEX II
Quality system assessment
1.
|
In the case of full quality assurance system, the verification should ascertain that the application of the quality system assures the conformity of the devices (1) with the legal requirements set out in Directive 90/385/EEC, Directive 93/42/EEC and Directive 98/79/EC. In the case of production or product quality assurance, the verification should ascertain that the application of the quality system ensures the conformity of the devices with the device type (2).
|
2.
|
The quality system assessment should include audits on the premises of the manufacturer and, if this is also necessary to ensure efficient control, on those of its critical subcontractors or of its crucial suppliers. Notified bodies should establish a risk-based approach to identify such subcontractors and suppliers and should clearly document this decision process.
|
3.
|
Notified bodies should identify which products the manufacturer regards as covered by its application, whether these products fall under Directive 90/385/EEC, Directive 93/42/EEC and Directive 98/79/EC and whether there have been changes to these products or to the quality system since the last audit or since the application. Furthermore, notified bodies should identify the post-market information available to them or to the manufacturer, which might need to be taken into account when planning and executing the audit.
|
4.
|
For medical devices of Class IIa or IIb, the notified bodies should review the technical documentation on the basis of representative samples with a frequency and depth following established best practices, taking account of the device’s class, risk and novelty. Samples chosen and reviews conducted should be clearly documented and justified. Over the period of certification of the specific quality system (i.e. for a maximum of five years) the sampling plan should be sufficient to ensure that every device category covered by the certificate has been sampled. Where doubts arise as to the conformity of a device, including its documentation, notified bodies should carry out or ask for relevant tests of the device. Where any non-conformity of a device is detected, they should investigate whether elements of the quality system or incorrect application thereof caused the non-conformity. Where a test has been carried out, notified bodies should provide the manufacturer with a test report and with an audit report which highlights in particular the link between quality system deficiencies and detected non-conformities of devices.
|
5.
|
Notified bodies should verify whether the quality objectives and the quality manual or procedures developed by the manufacturer are appropriate to ensure the conformity of the devices falling under the application of the manufacturer.
|
6.
|
Notified bodies should verify whether the manufacturer’s business organisation is appropriate for ensuring the conformity of the quality system and of the medical devices. In particular, the following aspects should be examined: the organisational structure, the qualification of managerial staff and their organisational authority, the qualification and the training of other staff, the internal auditing, the infrastructure, and the monitoring of the quality system in operation, including with regard to involved third parties such as suppliers or subcontractors.
|
7.
|
Notified bodies should verify the existence of an unequivocal product identification system. This system should ensure that the notified body’s certificates, the manufacturer’s declarations of conformity and the manufacturer’s technical documentations can, in conjunction with that system, unequivocally be attributed to certain devices and not to others.
|
8.
|
Notified bodies should verify the manufacturer’s procedures with regard to the product documentation. The procedures relating to the product documentation should ensure that all products intended to be placed on the market or put into service are covered by the necessary certificates issued or to be issued by the notified body. The procedures with regard to the product documentation should also ensure that all products intended to be placed on the market or put into service, regardless of their trade name, are covered by the declarations of conformity of the manufacturer and that these are contained in and are compatible with the technical documentation. Notified bodies should verify the correct execution of these procedures by sampling the product documentation of individual devices.
|
9.
|
Notified bodies should verify that the manufacturer’s procedures aiming at the fulfilment of procedural legal requirements, in particular with regard to determining the appropriate class and conformity assessment procedure, are up-to-date, complete, consistent and correct. These procedures should take account of the necessity to provide data in order to allow the notified bodies to respect their consultation obligations for certain devices referred to in Section 1 of Annex I.
|
10.
|
Notified bodies should verify that the manufacturer’s procedures aiming at the fulfilment of device related legal requirements, are up-to-date, complete, consistent and correct. They should verify that the procedures on the risk management are in conformity with the legal requirements contained in Part I (general requirements) of Annex 1 to Directive 90/385/EEC, in Part I of Annex I to Directive 93/42/EEC and in Part A of Annex I to Directive 98/79/EC and that the procedures cover among others the aspects listed in Section 4 of Annex I to this Recommendation. They should verify the correct execution of these procedures by sampling the product documentation of individual devices.
|
11.
|
In case of manufacturers of medical devices other than in vitro diagnostic devices, notified bodies should verify that the manufacturer’s procedures on clinical evaluations and on the post-market clinical follow-up are complete and correct and that these are correctly implemented. To that end, they should examine clinical evaluations and the post-market clinical follow-up for some of the device types covered by the application, applying the principles described in Section 5 of Annex I to this Recommendation. They should verify the correct execution of these procedures by sampling the product documentation of individual devices.
|
12.
|
In case of manufacturers of in vitro diagnostic medical devices, notified bodies should verify the manufacturer’s working procedures on performance evaluations, on the identification of certified reference materials or reference measurement procedures to allow for metrological traceability. They should verify the correct execution of these procedures by sampling the product documentation of individual devices.
|
13.
|
Notified bodies should verify that the procedures regarding the design and product development, including any change control procedures, are appropriate to ensure the compliance of the devices.
|
14.
|
Notified bodies should verify that the manufacturer controls the manufacturing environment and processes so as to ensure the conformity of the devices with the legal requirements. Notified bodies should pay special attention to critical processes such as design control, establishment of material specifications, purchasing and control of incoming material or components, assembling, software validation, sterilisation, batch-release, packaging, and product quality control, regardless of whether they are subcontracted or not.
|
15.
|
Notified bodies should verify the manufacturer’s system ensuring traceability of materials and components, from the entry into the manufacturer’s, suppliers’ or subcontractors’ premises to the delivery of the final product. In particular where risks might be caused by the exchange of raw materials, notified bodies should check the coherence between the quantity of produced or purchased crucial raw material or components approved for the design and the quantity of finished products.
|
16.
|
Notified bodies should verify that experience gained in the post-production phase, in particular user complaints and vigilance data, is systematically collected and evaluated for the devices covered by the application of the manufacturer and that the necessary improvement of the devices or of their production has been initiated. They should in particular verify that the manufacturer has in place distributor, user or patient related business processes which are suitable for providing information indicating the need for reviewing the design of the device, its manufacturing or the quality system.
|
17.
|
Notified bodies should verify that the documentation and records with regard to the quality system and its changes, the procedure of management review, and the respective documentation control are up-to-date, consistent, complete, correct and properly structured.
|
18.
|
At each annual surveillance audit, the notified bodies should verify that the manufacturer correctly applies the approved quality management system and the post-market surveillance plan.
|
19.
|
The notified body should clearly document the conclusions of its assessment and it should be clearly evidenced how the conclusions are taken into account as part of the notified body’s decision making process.
|
General advice in case of outsourcing of the production via subcontractors or suppliers
Critical subcontractors or crucial suppliers may be suppliers of suppliers or even suppliers further down the supply chain. Notified bodies should refrain from signing arrangements with manufacturers unless they receive access to all critical subcontractors and crucial suppliers and thus to all sites where the devices or its crucial components are produced, regardless of the length of the contractual chain between the manufacturer and the subcontractor or supplier.
Notified bodies should note that manufacturers:
(a)
|
have to fulfil their obligations themselves regardless of any partial or total outsourcing of the production via subcontractors or suppliers;
|
(b)
|
do not fulfil their obligation to have at their disposal the full technical documentation and/or of a quality system by referring to the technical documentation of a subcontractor or supplier and/or to their quality system;
|
(c)
|
should integrate the quality system of critical subcontractors and of crucial suppliers with their quality system;
|
(d)
|
need to control the quality of services provided and of components supplied and the quality of production thereof regardless of the length of the contractual chain between the manufacturer and the subcontractor or supplier.
|
(1) See first sentence of Section 3.2 of Annex 2 to Directive 90/385/EEC, first sentence of Section 3.2 of Annex II to Directive 93/42/EEC and first sentence of Section 3.2 of Annex IV to Directive 98/79/EC.
(2) See first sentence of Section 3.2 of Annex 5 to Directive 90/385/EEC, first sentence of Section 3.2 of Annex V and first sentence of Section 3.2 of Annex VI to Directive 93/42/EEC and first sentence of Section 3.2 of Annex VII to Directive 98/79/EC.
ANNEX III
Unannounced audits
1.
|
Notified bodies should carry out unannounced audits at least once every third year. Notified bodies should increase the frequency of unannounced audits if the devices bear a high risk, if the devices of the type in question are frequently non-compliant or if specific information provides reasons to suspect non-conformities of the devices or of their manufacturer. The timing of the unannounced audits should be unpredictable. As a general principle an unannounced audit should not take less than one day and should be executed by at least two auditors.
|
2.
|
Notified bodies may, instead of or in addition to visiting the manufacturer, visit one of the premises of the manufacturer’s critical subcontractors or crucial suppliers if this is likely to ensure more efficient control. This applies in particular if the main part of the design development, manufacturing, testing or another crucial process is located with the subcontractor or supplier.
|
3.
|
Within the context of such unannounced audits, the notified bodies should check a recently produced adequate sample, preferably a device taken from the ongoing manufacturing process, for its conformity with the technical documentation and with legal requirements. The check of the conformity of the device should include the verification of the traceability of all critical components and materials and of the manufacturer’s traceability system. The check should encompass a file review and, if necessary in order to establish the conformity, a test of the device.
To prepare the test, notified bodies should request from the manufacturer all the relevant technical documentation including previous test protocols and results. The test should be undertaken in accordance with the testing procedure defined by the manufacturer in the technical documentation which has to be validated by the notified body. The test may also be performed by the manufacturer, its critical subcontractor or crucial supplier under observation of the notified body.
|
4.
|
Notified bodies in charge of product assessment (1) should, in addition to the steps foreseen in Sections 1, 2 and 3, sample devices belonging to at least three different device types and, where the manufacturer produces more than 99 device types, devices belonging to at least every hundredth type at the end of the production chain or in the manufacturer’s warehouse with a view of testing the conformity of the device types. Variants containing a technical difference which might affect safety or performance of the device should be counted as a separate device type. Dimensional size variants should not be regarded as different types unless specific risks are linked to the dimension. These samples should be tested by the notified bodies or by qualified personnel under their observation on their own premises, or on the manufacturer’s premises, or on the premises of the manufacturer’s critical subcontractor or crucial supplier or in external laboratories. Sampling criteria and testing procedures should be defined in advance. In particular if a sampling in the manufacturer’s premises is not possible, notified bodies should take samples from the market, if necessary with support by the competent authorities, or should perform testing on a device installed at a customer location. To prepare the test, notified bodies should request from the manufacturer relevant technical documentation including final batch testing reports, previous test protocols and results.
|
5.
|
Notified bodies in charge of verifying the quality system of the manufacturer (2) should, in addition to the steps foreseen in Sections 1, 2 and 3, verify whether the manufacturing activity ongoing at the time of the unannounced audit is in line with the manufacturer’s documentation relevant for the manufacturing activity and that both are in conformity with legal requirements. In addition, these notified bodies should check in more detail at least two critical processes such as design control, establishment of material specifications, purchasing and control of incoming material or components, assembling, sterilisation, batch-release, packaging, or product quality control. Amongst the suitable critical processes, notified bodies should select one which has a high likelihood of non-conformity and one which is particularly safety relevant.
|
General advice with regard to contractual arrangements between the notified body and the manufacturer for the organisation of unannounced audits
In order to ensure that notified bodies are in a position to perform unannounced audits, some modalities, such as the following ones, should be considered.
Unannounced audits in premises of the manufacturer or its critical subcontractors or crucial suppliers should be foreseen in the contractual arrangements between the notified bodies and the manufacturers. If a visa is needed to visit the country where the manufacturer is located, the contractual arrangements should contain, as an annex, an invitation to visit the manufacturer at any time and an invitation which leaves the date of signature and the date of visit open (to be filled-in by the notified body). The contractual arrangements should also contain, as an annex, similar invitations issued by the critical subcontractors or crucial suppliers.
The contractual arrangements should foresee that the manufacturers continuously inform the notified bodies on the periods when devices falling under the notified bodies’ certificates will not be manufactured. The contractual arrangements should authorise the notified bodies to end the contract as soon as their permanent unannounced access to the premises of the manufacturer or its critical subcontractors or crucial suppliers is no longer assured.
The contractual arrangements should furthermore cover the measures to be taken by notified bodies to ensure the security of their auditors. The contractual arrangements should provide for a financial compensation for the unannounced audits including, where applicable, the device acquisition, its testing and security arrangements.
(1) According to Section 2(a) and Annex I to this Recommendation.
(2) According to Section 2(b) and Annex II to this Recommendation.
Iscriviti a:
Post (Atom)